What is LED?
An LED, or Light-Emitting Diode, is a semiconductor device that emits light when an electric current passes through it. Due to their efficiency, longevity, and versatility, LEDs are widely used in various applications.
LED Symbol

The symbol for an LED in circuit diagrams is similar to a standard diode symbol but includes two small arrows pointing away from the diode, representing the emission of light. This helps distinguish LEDs from other types of diodes.
LED Construction
Basic Structure
- Semiconductor Material: Typically made from materials such as gallium arsenide (GaAs), gallium phosphide (GaP), or gallium nitride (GaN).
- P-N Junction: The LED has a p-type semiconductor (with holes) and an n-type semiconductor (with electrons), forming a p-n junction where light emission occurs.
- Encapsulation: LEDs are usually encapsulated in clear or colored plastic to protect the semiconductor material and help focus the light.
LED Working
An LED, or Light Emitting Diode, operates on the principles of electroluminescence within a semiconductor material. The core structure of an LED involves a p-n junction made from two types of semiconductor materials: p-type and n-type. The p-type material contains positive charge carriers known as holes, while the n-type material contains negative charge carriers known as electrons.
When a forward voltage is applied to the LED, with the positive terminal connected to the p-type material and the negative terminal connected to the n-type material, the electrons and holes are pushed toward the p-n junction. At this junction, electrons from the n-type material recombine with holes in the p-type material. This recombination process releases energy in the form of photons, which produce light. The specific color of the light depends on the energy band gap of the semiconductor materials used. For instance, gallium arsenide (GaAs) emits infrared light, gallium phosphide (GaP) can emit red, green, or yellow light, and gallium nitride (GaN) emits blue light.
The emission of light in an LED is a result of the electrons moving from a higher energy state in the conduction band of the n-type material to a lower energy state in the valence band of the p-type material. This transition releases energy that is directly converted into light. The efficiency of this process makes LEDs highly effective at converting electrical energy into light with minimal heat production, unlike traditional incandescent bulbs, which lose a significant amount of energy as heat.
LEDs are encapsulated in a clear or colored epoxy resin, which protects the delicate semiconductor material and helps focus the light output. This encapsulation can also affect the light’s color and intensity, allowing for a wide range of applications, from simple indicator lights to complex display screens and general illumination.
The forward voltage applied to an LED typically ranges from 1.8 to 3.3 volts, depending on the material and the desired color. The current passing through the LED must be carefully controlled using a current-limiting resistor or a constant current driver to prevent damage from excessive current.
What Determines the Colour of an LED?
The color of an LED is primarily determined by the semiconductor material used in its construction and the energy band gap of that material. The energy band gap is the difference in energy between the conduction band and the valence band of the semiconductor. When an LED is forward-biased, electrons and holes recombine at the p-n junction, releasing energy as photons. The energy of these photons corresponds to the energy band gap, and this energy determines the wavelength and, thus, the color of the emitted light.
Different semiconductor materials have different band gaps, leading to the emission of different colors of light. For instance, Gallium Arsenide (GaAs) has a band gap that results in the emission of infrared light. Gallium Phosphide (GaP) can produce red, green, or yellow light depending on its specific composition and doping. Gallium Nitride (GaN) has a band gap that results in blue or ultraviolet light.
The doping process, which involves adding impurities to the semiconductor material, can also affect the color by slightly altering the band gap and the recombination process. Additionally, for white LEDs, a blue LED is often coated with a phosphor material that converts some of the blue light into other colors, resulting in the perception of white light.
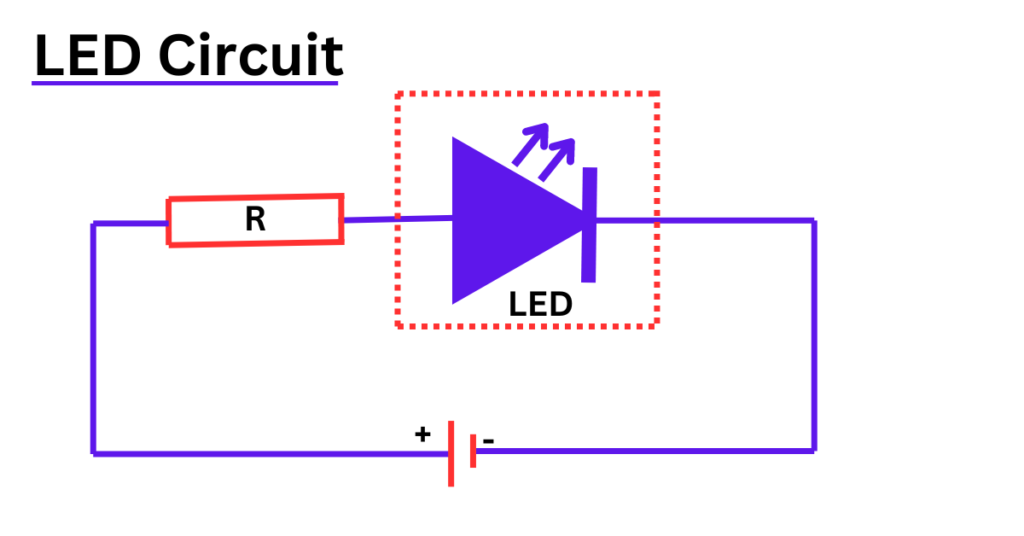
LED Circuit
To create a simple LED circuit, you will need a few basic components: an LED, a current-limiting resistor, a power supply (battery), and connecting wires. The circuit diagram of LED circuit is shown below,
Types of LED
- Standard LEDs: Used for indicators and displays.
- High-Brightness LEDs: Used in lighting and displays requiring high illumination.
- RGB LEDs: Can produce various colors by combining red, green, and blue light.
- Organic LEDs (OLEDs): Used in advanced display technologies for televisions, smartphones, and monitors.
- Infrared LEDs: Emit infrared light, commonly used in remote controls and security systems.
LED Applications
- Indicator Lights: These are commonly used in electronic devices to indicate status.
- Displays: These are used in digital displays for devices like calculators, clocks, and signage.
- Lighting: Increasingly used for general lighting in homes, offices, and streetlights due to their efficiency.
- Automotive Lighting: Used in headlights, brake lights, and interior lighting.
- Televisions and Monitors: These are used on screens for their bright and energy-efficient display capabilities.
Advantages of LED over Incandescent Lamps
- Energy Efficiency: LEDs consume significantly less power compared to incandescent lamps.
- Longevity: LEDs have a much longer lifespan, often lasting tens of thousands of hours.
- Durability: LEDs are more robust and resistant to shocks and vibrations.
- Lower Heat Emission: LEDs produce minimal heat, making them safer and more efficient.
- Environmental Impact: LEDs do not contain harmful substances like mercury and are more environmentally friendly.
Frequently Asked Question-FAQs
- What is the main difference between LEDs and traditional incandescent bulbs?
- LEDs consume less energy, last longer, and generate less heat than incandescent bulbs.
- Can LEDs be used with dimmer switches?
- Yes, but it’s important to use LEDs that are specifically labeled as dimmable and compatible with your dimmer switch.
- Do LEDs produce UV light?
- Standard LEDs do not produce significant UV light, making them safer for use in homes and offices.
- Why are LEDs considered environmentally friendly?
- LEDs are energy-efficient, have a long lifespan, and do not contain hazardous materials like mercury.
- Can LEDs be used in outdoor applications?
- Yes, LEDs are commonly used in outdoor lighting due to their durability and efficiency. Make sure they are designed for outdoor use to endure various weather conditions.